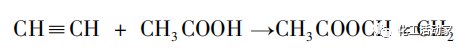Vinyl acetate (VAc), also known as vinyl acetate or vinyl acetate, is a colorless transparent liquid at normal temperature and pressure, with a molecular formula of C4H6O2 and a relative molecular weight of 86.9. VAc, as one of the most widely used industrial organic raw materials in the world, can generate derivatives such as polyvinyl acetate resin (PVAc), polyvinyl alcohol (PVA), and polyacrylonitrile (PAN) through self polymerization or copolymerization with other monomers. These derivatives are widely used in construction, textiles, machinery, medicine, and soil improvers. Due to the rapid development of the terminal industry in recent years, the production of vinyl acetate has shown a trend of increasing year by year, with the total production of vinyl acetate reaching 1970kt in 2018. Currently, due to the influence of raw materials and processes, the production routes of vinyl acetate mainly include acetylene method and ethylene method.
1、Acetylene process
In 1912, F. Klatte, a Canadian, first discovered vinyl acetate using excess acetylene and acetic acid under atmospheric pressure, at temperatures ranging from 60 to 100 ℃, and using mercury salts as catalysts. In 1921, German CEI Company developed a technology for the vapor phase synthesis of vinyl acetate from acetylene and acetic acid. Since then, researchers from various countries have continuously optimized the process and conditions for the synthesis of vinyl acetate from acetylene. In 1928, Hoechst Company of Germany established a 12 kt/a vinyl acetate production unit, realizing industrialized large-scale production of vinyl acetate. The equation for producing vinyl acetate by the acetylene method is as follows:
Main reaction:
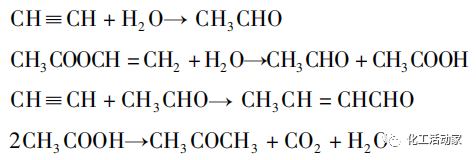
Acetylene method is divided into liquid phase method and gas phase method.
The reactant phase state of the acetylene liquid phase method is liquid, and the reactor is a reaction tank with a stirring device. Due to the shortcomings of liquid phase method such as low selectivity and many by-products, this method has been replaced by acetylene gas phase method at present.
According to the different sources of acetylene gas preparation, the acetylene gas phase method can be divided into natural gas acetylene Borden method and carbide acetylene Wacker method.
The Borden process uses acetic acid as an adsorbent, which greatly improves the utilization rate of acetylene. However, this process route is technically difficult and requires high costs, so this method occupies an advantage in areas rich in natural gas resources.
The Wacker process utilizes acetylene and acetic acid produced from calcium carbide as raw materials, using a catalyst with activated carbon as carrier and zinc acetate as active component, to synthesize VAc under atmospheric pressure and reaction temperature of 170~230 ℃. The process technology is relatively simple and has low production costs, but there are shortcomings such as easy loss of catalyst active components, poor stability, high energy consumption, and large pollution.
2、Ethylene process
Ethylene, oxygen, and glacial acetic acid are three raw materials used in the ethylene synthesis of vinyl acetate process. The main active component of the catalyst is typically the eighth group noble metal element, which is reacted at a certain reaction temperature and pressure. After subsequent processing, the target product vinyl acetate is finally obtained. The reaction equation is as follows:
Main reaction:
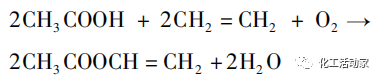
Side effects:
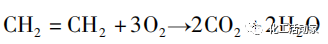
The ethylene vapor phase process was first developed by Bayer Corporation and was put into industrial production for the production of vinyl acetate in 1968. Production lines were established in Hearst and Bayer Corporation in Germany and National Distillers Corporation in the United States, respectively. It is mainly palladium or gold loaded on acid resistant supports, such as silica gel beads with a radius of 4-5mm, and the addition of a certain amount of potassium acetate, which can improve the activity and selectivity of the catalyst. The process for the synthesis of vinyl acetate using ethylene vapor phase USI method is similar to Bayer method, and is divided into two parts: synthesis and distillation. The USI process achieved industrial application in 1969. The active components of the catalyst are mainly palladium and platinum, and the auxiliary agent is potassium acetate, which is supported on an alumina carrier. The reaction conditions are relatively mild and the catalyst has a long service life, but the space-time yield is low. Compared to the acetylene method, the ethylene vapor phase method has greatly improved in technology, and the catalysts used in the ethylene method have continuously improved in activity and selectivity. However, the reaction kinetics and deactivation mechanism still need to be explored.
The production of vinyl acetate using the ethylene method uses a tubular fixed bed reactor filled with catalyst. The feed gas enters the reactor from the top, and when it contacts the catalyst bed, catalytic reactions occur to generate the target product vinyl acetate and a small amount of by-product carbon dioxide. Due to the exothermic nature of the reaction, pressurized water is introduced into the shell side of the reactor to remove the reaction heat by using the vaporization of water.
Compared with the acetylene method, the ethylene method has the characteristics of compact device structure, large output, low energy consumption, and low pollution, and its product cost is lower than that of the acetylene method. The product quality is superior, and the corrosion situation is not serious. Therefore, the ethylene method gradually replaced the acetylene method after the 1970s. According to incomplete statistics, about 70% of VAc produced by ethylene method in the world has become the mainstream of VAc production methods.
Currently, the most advanced VAc production technology in the world is BP’s Leap Process and Celanese’s Vantage Process. Compared to the traditional fixed bed gas phase ethylene process, these two process technologies have significantly improved the reactor and catalyst at the core of the unit, improving the economy and safety of unit operation.
Celanese has developed a new fixed bed Vantage process to address the problems of uneven catalyst bed distribution and low ethylene one-way conversion in fixed bed reactors. The reactor used in this process is still a fixed bed, but significant improvements have been made to the catalyst system, and ethylene recovery devices have been added in the tail gas, overcoming the shortcomings of traditional fixed bed processes. The yield of the product vinyl acetate is significantly higher than that of similar devices. The process catalyst uses platinum as the main active component, silica gel as the catalyst carrier, sodium citrate as a reducing agent, and other auxiliary metals such as lanthanide rare earth elements such as praseodymium and neodymium. Compared with traditional catalysts, the selectivity, activity, and space-time yield of the catalyst are improved.
BP Amoco has developed a fluidized bed ethylene gas phase process, also known as the Leap Process process, and has built a 250 kt/a fluidized bed unit in Hull, England. Using this process to produce vinyl acetate can reduce the production cost by 30%, and the space time yield of the catalyst (1858-2744 g/(L · h-1)) is much higher than that of the fixed bed process (700-1200 g/(L · h-1)).
The LeapProcess process uses a fluidized bed reactor for the first time, which has the following advantages compared to a fixed bed reactor:
1) In a fluidized bed reactor, the catalyst is continuously and uniformly mixed, thereby contributing to the uniform diffusion of the promoter and ensuring a uniform concentration of the promoter in the reactor.
2) The fluidized bed reactor can continuously replace the deactivated catalyst with fresh catalyst under operating conditions.
3) The fluidized bed reaction temperature is constant, minimizing catalyst deactivation due to local overheating, thereby extending the service life of the catalyst.
4) The heat removal method used in the fluidized bed reactor simplifies the reactor structure and reduces its volume. In other words, a single reactor design can be used for large-scale chemical installations, significantly improving the scale efficiency of the device.
Post time: Mar-17-2023