Product Name:Methyl Ethyl Ketone
Molecular format:C4H8O
CAS No:78-93-3
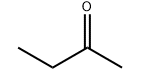
Product molecular structure:
Specification:
|
Item |
Unit |
Value |
|
Purity |
% |
99.8min |
|
Color |
APHA |
8max |
|
Acid value(as acetate acid) |
% |
0.002max |
|
moisture |
% |
0.03max |
|
Appearance |
- |
Colorless liquid |
Chemical Properties:
Methyl ethyl ketone is susceptible to various reactions because of its carbonyl group and the active hydrogen adjacent to the carbonyl group. Condensation occurs when heated with hydrochloric acid or sodium hydroxide to produce 3,4-dimethyl-3-hexen-2-one or 3-methyl-3-hepten-5-one. When exposed to sunlight for a long time, ethane, acetic acid and condensation products are produced. Generate diacetyl when oxidized with nitric acid. When oxidized with strong oxidizing agents such as chromic acid, acetic acid is generated. Butanone is relatively stable to heat, and thermal cleavage at higher temperatures produces enone or methyl enone. When condensed with aliphatic or aromatic aldehydes, high molecular weight ketones, cyclic compounds, ketone condensation and resins are produced. For example, condensation with formaldehyde in the presence of sodium hydroxide first produces 2-methyl-1-butanol-3-one, followed by dehydration to methacrylatone.
Resinization occurs upon exposure to sunlight or UV light. Condensation with phenol yields 2,2-bis(4-hydroxyphenyl)butane. Reacts with aliphatic esters in the presence of a basic catalyst to produce β-diketones. Acylation with acidic anhydride in the presence of an acidic catalyst to form β-diketones. Reacts with hydrogen cyanide to form cyanohydrin. Reacts with ammonia to form ketopiperidine derivatives. The α-hydrogen atom of butanone is readily substituted with halogens to form various halogenated ketones, such as 3-chloro-2-butanone by interaction with chlorine. Interaction with 2,4-dinitrophenylhydrazine produces yellow 2,4-dinitrophenylhydrazone.
Application:
Methyl ethyl ketone (2-butanone, ethyl methyl ketone, methyl acetone) is an organic solvent of relatively low toxicity, which is found in many applications. It is used in industrial and commercial products as a solvent for adhesives, paints, and cleaning agents and as a de-waxing solvent. A natural component of some foods, methyl ethyl ketone can be released into the environment by volcanoes and forest fires.It is used in themanufacture of smokeless powder and colorless synthetic resins, as a solvent, and insurface coating. It is also used as a flavoringsubstance in food.
MEK is used as a solvent for various coating systems, for example, vinyl, adhesives, nitrocellulose, and acrylic coatings. It is used in paint removers, lacquers, varnishes, spray paints, sealers, glues, magnetic tapes, printing inks, resins, rosins, cleaning solutions, and for polymerization. It is found in other consumer products, for example, household and hobby cements, and wood-filling products. MEK is used in dewaxing lubricating oils, the degreasing of metals, in the production of synthetic leathers, transparent paper and aluminum foil, and as a chemical intermediate and catalyst. It is an extraction solvent in the processing of foodstuffs and food ingredients. MEK can also be used to sterilize surgical and dental equipment.
In addition to its manufacture, environmental sources of MEK include exhaust from jet and internal combustion engines, and industrial activities such as gasification of coal. It is found in substantial amounts in tobacco smoke. MEK is produced biologically and has been identified as a product of microbial metabolism. It has also been found in plants, insect pheromones, and animal tissues, and MEK is probably a minor product of normal mammalian metabolism. It is stable under ordinary conditions but can form peroxides on prolonged storage; these may be explosive.
Products categories
-

Phone
-

E-mail
-

Whatsapp
-

Top













